Lecture 19 - Atoms and Light (2/26/96)
Seeds: Chapters 5, 6
- Interference and Interferometry
- In the previous lecture, I mentioned that the resolution
of a telescope was due to the wave packets of the photons
being cut off by the telescope. I also showed examples of
separate telescopes linked together as interferometers which
gave the resolving power of a single telescope the size of the
distance between the telescopes. These two things are related,
and can be understood by considering the interference of
different parts of the light or radio wave corresponding to a
photon.
- The resolution of a telescope is determined by the ability of
the telescope to interfere the parts of the wavefront of
light coming from an angle to localize the direction.
- As an analogy, the way you can tell what direction a wave is
coming from at the shore of the beach is to notice the timing
of arrival of the peaks and troughs of the wave as they hit
the beach. If you could look over a long stretch of the shore,
you could easily see small differences between angles of arrival,
because the equivalent differences in arrival times at large
separations on the beach are proportionally large. If you had
only a small section of beach to check, it would be hard to notice
even large differences in angle.
- A telescope can be thought of as a wave-catcher as well as a photon
bucket. (Again, the wave-particle duality.) In fact, the whole
purpose of the parabolic surface of the primary mirror (or the
curved surfaces of the objective lens) is to arrange that the various
portions of an incoming plane-parallel photon wavefront reach the
focus at the same time!
- A wavefront perpendicular to the optical axis of the telescope
is arranged to arrive at the focus in phase, that is, all
parts of the wavefront at the same phase of the sinusoid arrive
at the same time, and add together. In the focal plane, but off
the focus, wavefronts at different angles are arranged to arrive
in phase. This is how an image is made.
- It is useful to define an aperture plane as a plane
perpendicular to the optical axis of the telescope (at the
top of the telescope - the aperture that is - though any such
plane will
do). All parts of the wave arriving simultaneously at the
aperture are added up at the focus. Thus, it is easy to
see maximum brightness at the focus occurs for on-axis waves.
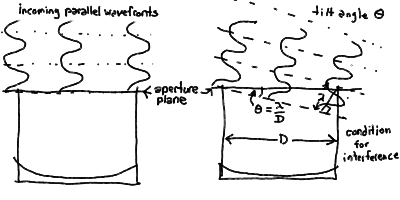
- Note that if we tilt the incoming wavefronts at a slight angle,
the various parts no longer add up at the focus in phase, but
slightly out of phase. In fact, if we tilt it far enough,
the wave striking the aperture plane at the intersection with
the optical axis are exactly 180 degrees out of phase with the
part of the wave striking the aperture at the edge of the
telescope at the same time, and thus cancel out at the focus.
When you add up the contributions for all the parts of the aperture,
you get a greatly reduced brightness as the different phases
interfere. At this angle in fact, you can see that all points
along this cut of the aperture are paired with another point 180
degrees out of phase, so we would expect zero brightness at the
focus.
- You can use the small angle formula to see that this occurs when
the diameter times the angle (in radians) equals one wavelength.
This is just our formula for the resolution! (See the diagram below).
- Since we are dealing with plane waves from an infinitely distant
astronomical source, symmetry shows us that for this special angle
we would get zero signal for a square telescope aperture
in the same direction. It turns out that for a circular aperture
you need to tilt a little bit farther to get exactly zero signal
(because there is more in the center than the edge) - a factor of
about 1.22 to be precise. For our purposes,
resolution = wavelength/diameter will do just fine.
- For angles less than the critical angle, the brightness is diminished
but not zero. Thus, a star offset from an on-axis star by such a
small angle will be blurred together. This is what we mean by
"resolution".
- You notice that when adding up the wavefront, we do it on a point
by point basis in the aperture plane. We can interfere bits of
the aperture with each other by placing a mask at the top
of the telescope, for example two small holes at opposite edges.
Our interference calculations would show in this case that waves
tilted by one wavelength at the separation between them would add
up in phase with full brightness, and angles tilted by one half
wavelength would add up out of phase with zero brightness, with
a smooth sinusoidal gradation in between. In fact, our response
pattern of angles on the sky would follow a sine function! The
resolution of our little interferometer is given by the
width between zero response (or between the maximum responses) which
is just our prevouse angle = wavelength/separation.
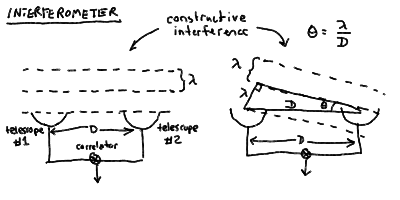
- There is in fact no particular reason that we need the rest of
the aperture in this case - we can make our interferometer out
of separate telescopes as long as we can arrange to have the
waves from each arrive at the "focus" simultaneously to interfere.
- Two telescopes (or radio antennas) separated by some distance D
make an interferometer of baseline D. The "focus" where
the waves are combined is called a correlator, and it can
be separate from either telescope.
- A whole bunch of telescopes linked together to form an interferometer
is called an interferometer array. We can see that such an
array has the equivalent resolution of the longest baseline
(separation) in the array. The angular response from a single
baseline is a sinusoid on the sky (with angular wavelength of
photon wavelength/D), but by making an array with more and more
different baseline lengths and orientations we approach the single
telescope response shape (which is a circularly symmeteric pattern
for a circular aperture).
- Though the resolution is of a telescope with the diameter of the
longest baseline, the light gathering power is merely the sum of
the individual telescope areas. You only get the photons you
collect.
- The Very Large Array (VLA), shown in the previous lecture, is an
array of 27 telescopes, each 25 meters in diameter, spread over
the New Mexico plains with baselines up to 36 km. The wave signals
from each antenna are piped to the central correlator using
waveguides (special metal pipes) buried under the desert.
- The Very Long Baseline Array (VLBA), on the other hand, is an array
of 9 antennas (again each 25 meters in diameter) located across the
US from Hawaii to the Virgin Islands. The maximum baseline is about
8612 km, giving superb resolution even at radio wavelengths
(for wavelength of 6cm, a typical VLBI observing wavelength,
this gives a resolution of 206265" x 0.06m/8612000m = 0.0014"!).
- Note that it is impractical to wire these telescopes together to
allow correlation of the signals, so the signals are recorded on
special magnetic tapes, which are taken later to a central facility
(in New Mexico) where they are played back together and correlated.
This actually works!
- Japanese scientists are building an orbiting radio telescope to be
used for VLBI. It is called VLBI Space Observatory Program (VSOP),
and should be launched in the next few years. In combination with
the Earth-bound VLBA, it will give baselines of up to 2.6 Earth
diameters (about 33000 km), which at 6cm wavelength gives a resolution
of 0.0004". The signals received at the satellite antenna are beamed
to Earth tracking stations where they are recorded then sent to the
VLBA center for correlation.
- Detectors (a brief intro)
- After light is collected and focused by the telescope, some sort
of detector is needed at the focus to turn the light into
information. Generally speaking, you can do one of two things:
detect photons, or detect waves.
- In the optical part of the spectrum, wavelengths are very small
(100 - 1000 nm) while photons are energetic, so photon detection
is the preferred method. Photons can be detected by inducing a
chemical reaction on a photographic plate (like film in your
35mm camera), or by knocking electrons around in a small silicon
wafer that looks much like a computer chip called a
charge-coupled device (CCD). There are CCD's in most video
cameras nowadays.
- With the development of CCD's, photographic plates are becoming more
and more rare (except for special applications like wide-field sky
surveys). The images made on CCD's are digital, and can be easily
computer-processed and stored. All of the Hubble Space Telescope
images were taken with CCDs.
- Because radio waves are large (0.1-1 meter), but radio photons are
weak, it is generally much easier to detect radio waves than photons.
Radio receivers work by causing the electric field in the wave
to induce a current in a wire, which is then amplified by a transistor
just like your home radio or TV (though using special devices that
are much more powerful because the signals are very weak). Because
the wave is detected, these receivers are well suited for
interferometry.
- Some radio detectors absorb the very weak photons and turn them into
heat energy in special wafers of silicon cooled to very nearly
absolute zero temperature. These are known as bolometers and
have the advantage that they can detect radiation over a wide spectrum,
since all the photons are turned into heat regardless of wavelength.
- If it is desired to examine the individual wavelengths of light, then
a dispersive element such as a prism is placed in front of the
detector. This makes what is called a spectrograph. In the
radio wavelengths, narrow-band filters are used instead of dispersive
elements (because it is hard to disperse long-wavelength waves in a
small space) to block out the unwanted wavelengths.
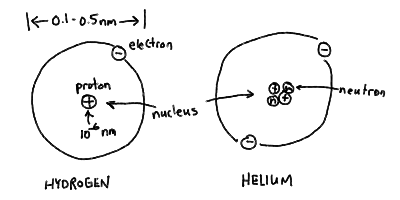
- The Atom
- Atoms are made up of subatomic particles: electrons, protons, and
neutrons. Electrons, light particles that carry negative
charge, were discovered by J.J. Thomson in 1897, and Ernest Rutherford
discovered in 1911 that the positive charge in the atom was
concentrated in a tiny nucleus. Later, the nucleus was shown
to contain positive protons, and charge neutral neutrons.
- In Rutherford's model of the atom, the nucleus is surrounded by
a cloud of electrons constantly in motion.
- Protons and neutrons are the most massive subatomic particles, though
even they are tiny. Both have about the same mass: 1.67 x 10^-24 g.
- Electrons are much less massive, with a mass of about 1/1836 of the
proton mass.
- Atoms are mostly empty space. The nucleus itself contains most of
the mass, but is only about 10^-6 nm ( 10^-15 m) in size! The
extent of the electron cloud is about 0.1 - 0.5 nm.
- The electric forces between the oppositely charged electrons and
protons hold the atom together. The protons and neutrons in the
nucleus are held together by the strong nuclear force, which can
overcome the mutual repulsion of the like-charged protons.
- Even though atoms are mostly empty space, two atoms don't easily
pass through each other because of the electric forces of the
electron clouds. The solidity of matter is purely the doing of
electric forces. When you press your hand against a tabletop,
it is actually the electron clouds in the atoms of your hand pushing
electrically against the electron clouds in the atoms of the table.
- Why don't all atoms just repel each other and fly apart, like a gas?
If you bring two atoms close enough together, with the right
configuration of electron clouds in each, then the two separate
atoms can merge, sharing electron clouds - this is called a
molecule.
- Molecules can fit together into solids (and to a lesser extent
liquids) by very very weak inter-molecular forces left over in
the outermost electron clouds. The physics of the attractive forces
between atoms and molecules is what makes chemistry work.
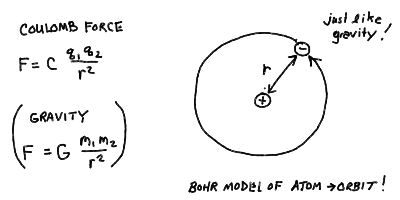
- Coulomb Forces and Electron Orbits
- The electric force, as specified by Charles Coulomb (1736-1806)
is given by the inverse-square law:
F = C q_1 q_2 / r^2
where
q_1 and q_2 are two electric charges, r the distance between them
and C a constant having to do with the units of charge (and distance).
- Notice any similarity to something we already know? Indeed, the
electric Coulomb force has the same dependence as the gravitational
force of Newton, with the charges standing in for masses.
- One difference with gravity is that electric charge can have both
positive and negative sign, so the electric force is either attractive
or repulsive. Opposite charges attract - like charges repel.
- The gravitational force, on the other hand, is strictly attractive.
There are no negative masses, and thus there is no gravitational
repulsion (anti-gravity).
- Since the electric and gravitational forces look the same
mathematically, then we might expect them to behave similarly
physically. Thus, we would expect that electrons would orbit
the nucleus much like a planet orbits the Sun!
- Just like it takes energy to send a spaceship out into a higher
orbit, and that if you take some of the energy of motion away
from a spaceship (say through friction with the atmosphere) it
will drop to a lower orbit, then so also does the energy of
the electron orbit rise with increasing distance from the nucleus.
- If we carry on our analogy with planets, then we might expect that
any orbit at all could be obtained for an electron, as long as
you gave it the proper energy.
- The energy of an electron can be changed by emitting or absorbing
a photon. This is, as we mentioned before, how the electromagnetic
force is transmitted, through the intermediation of photons. If
a photon is absorbed by the electron, then the outgoing electron
carries the energy and momentum of both the incoming electron and
photon. If an electron emits a photon, then its energy and momentum
are distributed between the outgoing electron and photon.
- Thus an electron in an atom can absorb a photon and jump to a higher
orbit, or emit a photon and fall to a lower orbit. The change in
orbit is given by the energy of the photon.
- There is one (at least one) conceptual problem with this. It was
known for quite a while that an accelerating electric charge radiates
electromagnetic radiation, that is, photons. This is how we use
an oscillating electric current to generate radio waves. This is also
why very hot things emit light - the moving atoms jiggle around the
electrons which emit photons. In any event, we would expect the
orbiting electrons, accelerated by the centripetal accleration of
the electrical force, to cause them to radiate photons and thus lose
energy and spiral in toward the nucleus. This was a big puzzle in
20th century physics.
- Quantization and Energy Levels
- Physics is a series of a few themes played over and over.
- It was mentioned before, in discussing the particle and wave nature
of a photon and light, that particles such as electrons have a
wave nature also. This was normally hard to detect, since the
wavelength was inversely proportional to the momentum of the particle.
Electrons can have wavelengths of less than a nanometer, and thus
can make useful electron microscopes that can see very tiny
things.
- It is obvious that in the tiny orbits in atoms, that we must take into
account the wave nature of the electron! That is, it is not an
orbiting particle like a planet that we must work with, but our task
is to fit an electron wave into a confined orbit in a way that makes
sense.
- According the the physicist de Broglie, particles
correspond to waves with wavelength given by
L = h / p
(where p is the momentum, and h is Planck's constant)
and frequency
f = E / h
(just like light).
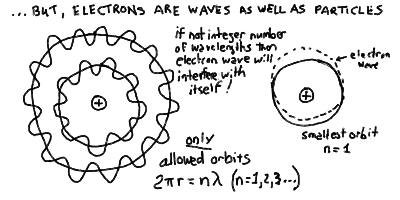
- What we have learned from dealing with light waves is that when you
bring parts of a wave together, then unless you are careful, it will
interfere with itself and cancel out. In fact, if you try
drawing a sinusoidal wave on a circlular orbit, then unless you adjust
the wavelength, you will arrive back at the starting point
out of phase and as you draw the wave around and around the
orbit, it will cancel out completely!
- Thus, you can see graphically that the only allowed wave orbits are
those with an integer number of wavelengths in the circumference
(see drawing below). Then, the wave wraps around and around always
in phase with itself - this sort of wave pattern is called a
standing wave. This is how a plucked string generates a
note of musical sound, it is allowed to oscillate only such that
a standing wave of motion is produced.
- Thus, we would expect that orbits are quantized
in a special way so that an integer number of standing electron
waves, say n, will fit on the circumference
2 Pi r = n
where the integer n = 1, 2, 3, ... and so on.
Thus we would expect only certain quantized energy
levels to exist in an atom, and thus only photons of special energies
corresponding to the differences between these levels to be absorbed
and emitted by the electrons.
- The different allowed orbits are roughly spherical (not circles) and
are called electron shells. Even though they are spheres,
not closed circles, our wave arguments still hold. The exact orbital
pattern of an electron wave determines the ability of an atom to
make molecules, because as you can see if it is concentrated into
a circle, then the positive proton can make its attractive force
felt in the perpendicular direction along the poles of the orbit!
It also turn out that we can pack in more than a single electron
in one shell. How many you can put in without them interfering with
each other is an important property of atomic structure.
We will discuss these topics more in the next lecture.
- The physics of the actions of wave-particles, and of quantized
states that arise when you work with them, is called quantum
mechanics. This subject was pioneered in the early parts of
this century, and has caused a revolution in our understanding of
the nature of the Universe.
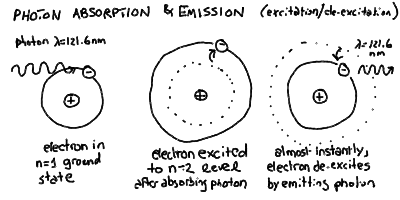
- So, in our quantum atom, an electron can absorb a photon of
specific energy and jump to a higher energy shell. However, it
turns out that it will almost immediately emit a photon and
jump back down to the original level, or one lower if possible,
emitting a photon in the process.
- You can see there is a minimum-energy orbit corresponding to
a single wavelength on the orbit. If we number the shells outward
from the nucleus by their integer number of waves, we would call
this the n = 1 orbit.
- Because rules of quantum mechanics allow us to only put a certain
number of electrons in each orbit, there is an atomic state where
all the lowest available orbits are filled. This is the lowest
energy state of the atom, and is called the ground state.
- If an atom is in the ground state, and one of its electrons absorbs
a photon and jumps to a higher-energy orbit, then we say that this
atom is now in an excited state. It almost immediately emits
a photon of the same energy and de-excites back to the ground
state. It would take a continuous bombardment by photons (or
collisions) to keep atoms in an excited state for any length
of time.
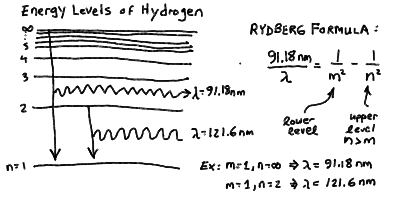
- What are the energy levels, and thus the allowed wavelengths of
emitted and absorbed photons, for a simple atom like hydrogen?
It turns out that there is a simple formula, called the Rydberg
formula, that gives this (see below).
- Note that there is a finite energy difference between the lowest orbit
and an electron orbiting at infinity. For hydrogen, this corresponds
to a wavelength of 91.18 nm. If a photon of this wavelength or shorter
is absorbed by an electron in the ground state, it will be knocked out
of the atom altogether! Note that a jump from the n = 1
to the n = 2 levels corresponds to a wavelength of 121.6 nm.
- In the next lecture, we will look in more detail at the energy
level emitted spectrum of the hydrogen atom.
- The Danish physicist Niels Bohr (1872-1962) devised a more or less
correct vision of the atom, though without the notion of waves or
quantum mechanics. This classical Bohr atom with quantized orbits
is often still used as a toy model.
Next Lecture - Atomic Spectra
Interference and Interferometry
Interference of wavefront at telescope causes blurring (resolution):
Interference between wavefronts received at separate telescopes makes
an interferometer work:
Detectors
The Atom
Structure of the atom - nucleus and electron "cloud" for hydrogen
and helium:
Coulomb Forces and Electron Orbits
The Bohr model of the atom - electrons as orbiting "planets" in an
atomic "solar system":
Quantization and Energy Levels
Packing the electron wave into orbit - standing waves and quantized
electron orbitals:
Orbitals correspond to different electron energy levels:
Jumping between these levels can be initiated by absorption of
a photon of the correct energy, but the atom quickly returns to
its de-excited state emitting a photon of the same energy:
Go to Previous Lecture ----
Go to Next Lecture
Back to the Lecture Notes Index
Back to the ASTR001/Sec3 Page
Steven T. Myers - Last revised 29Feb96







