Because we are first talking about transitions between different electronic energy levels in the same ionization state H I, we use the Boltzmann equation:
with statistical weight (the number of states available)
for each level of energy
Thus, we solve
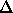 E21/kT ]
= 4 exp( - 118586/T )
E21/kT ]
= 4 exp( - 118586/T )
for E0 = 13.62 eV and
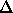 E21 = 13.62
( 1 - 1/4 ) eV = 10.22 eV.
E21 = 13.62
( 1 - 1/4 ) eV = 10.22 eV.
We take natural logarithms and rearrange to get
for the temperature at which the ground state (n=1) and first excited state (n=2) of H I are equally populated. Note that this temperature is much higher than those found typically in the atmospheres of stars, and thus to good aprroximation we can assume all neutral hydrogen is in the ground state (important later).
For the ionization states we use the Saha equation:
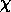 /kT ]
/kT ]
where the ionization potential from H I to H II is 13.62 eV, or
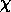 /k = 158114 K
/k = 158114 K
in terms of temperature. So we solve
or taking base-e logarithms and rearranging gives
or
which must be solved numerically. Inspection shows the solution should be near 10000 K. Like we did in the past, this equation can be solved by iteration, namely
starting with first guess T1=10000 K:
| i | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Ti | 10000 | 8353 | 8556 | 8528 | 8532 | 8532 |
and although convergence is not super-fast, it does the job. Note that at the moderate T = 8532 K, the H I is half ionized! Thus, by the time we have an appreciable population in the first excited state, most of the atoms are ionized to H II! Thus we are safe with the assumption that for reasonable temperature, all the H I is in the n=1 state.
Since we just showed that we can assume all H I is in the ground state, then
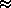 (N2/N1) /
(NII/NI + 1)
(N2/N1) /
(NII/NI + 1)
where
and
from problem 1.
To make things easy, we take logarithms
being careful to go from
in the final term.
We crank and tabulate:
| Type | T (K) | log(N2/N1) | log(NII/NI) | log(N2/N) |
|---|---|---|---|---|
| G0 | 6000 | -7.98 | -3.78 | -7.98 |
| F0 | 7200 | -6.55 | -1.67 | -6.56 |
| A0 | 10400 | -4.35 | 1.66 | -6.02 |
| B0 | 27000 | -1.31 | 6.76 | -8.06 |
As expected, we get maximum population (as a fraction of the total number of atoms) in the n=2 level for the A0 star. If we compare to Fig 8-13 of the text (ZG4 p.168) we find that our values for log(N_2/N_1) and log(N_II/N_I) agree, though the curve for log(N_2/N) peaks out at -5 instead of -6 as we found.
Since the Balmer absorption series are transitions from n=2 to n>2, strength is proportional to the number of atoms in the excited state, and thus should be strongest for A stars.
There are 3 ionization states of helium: He I (neutral, 2 electrons), He II (singly ionized, hydrogen-like), and He III (fully ionized). Since we are considering different ionization states, we use the Saha equation
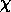 i/kT )
i=I,II -> II,III
i/kT )
i=I,II -> II,III
where we assume Pe = 2 Pa for the electron pressure. The ionization potentials are given:
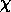 I = 24.5 eV
===>
I = 24.5 eV
===> 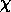 /kT = 284420/T
/kT = 284420/T 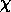 II = 54.4 eV
===>
II = 54.4 eV
===> 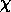 /kT = 631530/T
/kT = 631530/T but the tricky part is getting the partition functions Z:
| He I : | ( 2 electrons fill the 2 slots in the ground state ) | ===> ZI = 1 |
|---|---|---|
| He II : | ( 1 electron for the 2 slots in the ground state ) | ===> ZII = 2 |
| He III : | ( no electrons around the bare nucleus ) | ===> ZIII = 1 |
Note that since helium is even more extreme than hydrogen, it is almost certain to be in the ground state!
We tabulate:
| T (K) | log(NII/NI) | log(NIII/NII) |
|---|---|---|
| 100000 | 10.09 | 7.98 |
| 150000 | 10.94 | 9.33 |
| 200000 | 11.46 | 10.10 |
Actually, I grossly mis-estimated the interesting temperatures where the ionization transitions occur, and should have asked for:
| T (K) | log(NII/NI) | log(NIII/NII) |
|---|---|---|
| 10000 | -3.53 | -19.21 |
| 20000 | 3.40 | -4.74 |
| 30000 | 5.90 | 0.27 |
| 40000 | 7.24 | 2.87 |
We see that the helium is fully ionized by 40000~K, but will have a maximal population in the doubly ionized state at the crossover around 30000K, and thus the Helium II Balmer series might be present at this temperature. (Try this yourself.)
We wish to compute the length X over which
 ~
~ 
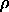 X = 1
X = 1
or

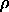 ) = 5.32 x 10-5 m
) = 5.32 x 10-5 m
and thus the "mean free path" for the photons in the Sun's core is 53 microns!
In a plane-parallel atmosphere with optical depth "tau" at zenith, we get the attenuation
 * sec(ZA) ]
* sec(ZA) ]
at zenith angle ZA from the vertical, where sec(ZA) = 1/cos(ZA) is also called the airmass since it measures the number of "atmospheres" along the path.
We have the intensities and magnitudes:
| above the atmosphere : | I = I0 | m = m0 |
|---|---|---|
| ZA=0°, sec(ZA)=1 : | I = I0 e- |
m = 3.0000 |
| ZA=60°, sec(ZA)=2 : | I = I0 e-2  |
m = 3.1086 |
where we have used sec(60)=2 airmasses. Note that if we take the intensity (or flux, for a given star solid angle) ratio

while from the magnitude difference we know
and thus
 = 100.0434
===>
= 100.0434
===>
 = 0.0434 ln(10) = 0.1
= 0.0434 ln(10) = 0.1
is the optical depth at zenith. If we then back out the effect of the atmosphere and express this as a magnitude difference dm
 =
10(3.000-m0)/2.5
=
10(3.000-m0)/2.5
but from above this is just
 = 100.0434
===>
(3.000-m0)/2.5 = 0.0434
= 100.0434
===>
(3.000-m0)/2.5 = 0.0434
or
and there is thus 0.1086 magnitudes of attenuation per atmosphere!
We use the perfect gas law
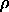 kT/µmH
kT/µmH
to get
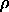 = µmHP/kT
= 1.13 kg m-3
= µmHP/kT
= 1.13 kg m-3
and for our optical depth of 0.1
 = 0.1 =
= 0.1 =

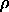 X
X
we get the opacity
 =
0.1/(
=
0.1/(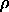 X) = 8.9 x 10-6
m2 kg-1
X) = 8.9 x 10-6
m2 kg-1
for an atmospheric thickness of X = 10 km.